To prepare for the health field in the New Visions program, I am studying the topic I detailed in my application: biofilm. Biofilms are persistent communities of microorganisms that are almost impossible to remove from a surface given enough time. Important in the fields of immunology and microbiology, it is essential to understand biofilms because they can help us identify their role in delaying wound healing and inflammation– problems that can pose serious health risks if not dealt with. It doesn’t only occur in the medical field; it can exist naturally throughout day-to-day life. Dog bowls, dirty bathtub corners, stones with moss near a waterfall, and plaques in our teeth are all examples of biofilm. Let’s dig deeper into how biofilm is formed in our next paragraph!
Biofilm can be strenuous to remove when given enough time, even with effective antibiotic treatments. During its initial attachment, free-floating planktonic cells in water with strong cellular walls attach to their surface when left untouched and given ideal environmental conditions. With this attachment, cells start expressing a matrix containing EPS (extracellular polymeric substances), which creates a slimy substance that helps with structure and protection. Settling on the attached surface, As they multiply and divide to spread, they disperse from one host to another during the last phase of their life cycle.
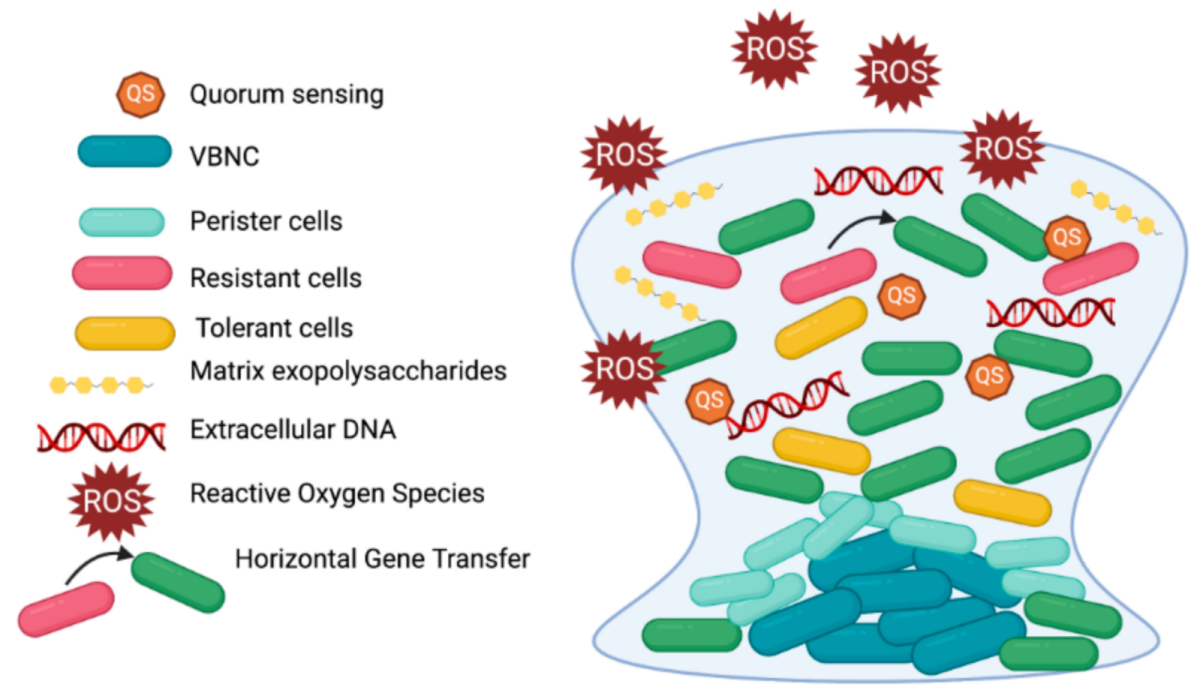
Within different biofilm communities, a metabolic cycle occurs where the waste of one organism acts as a nutrient for the other organisms. Anaerobic microorganisms, for instance, create anaerobic regions from their consumption of oxygen, an essential part of polymicrobial infections where both aerobic and anaerobic take place. Biofilm response to environmental stimuli is known as quorum sensing, a process allowing microorganisms to release and bind autoinducers (small diffusible molecules) which start a series of reactions based on their cell density. Genes associated with essentially beneficial functions of cells are activated with quorum sensing, taking place mostly between bacteria and eukaryote populations. The signaling molecules depend on two classifications: Gram-negative bacteria use N-acylated homoserine lactones to communicate, while gram-positive bacteria mainly utilize small peptides. When a limit for the amount of signaling molecules is reached, autoinducers bind to their specific receptor where signaling causes changes to gene expression, resulting in biological responses.
Unfortunately, biofilm can obtain tolerance to high doses of antibiotics. Reasons include the presence of metabolic inaction reducing the effect of antibiotics that target faster growth, slower antibiotic diffusion caused by EPS, and horizontal gene transfer. Layers of dead biofilm cells can also permanently anchor onto a surface, allowing more biofilm to grow. Traditional antibiotics unfortunately don’t have much effect in eradicating biofilm due to quick genetic changes within the biofilm cells, requiring alternative methods to remove these pesky biofilm populations. A proposed solution involves treating surfaces with a lubricant that infuses into tiny holes, attaching with capillary action. It was effective in destroying microorganism adhesion, showing that it can be used as a valid procedure of removal (Parker & Nina, et al.).
While delving into specific research papers during my sophomore year, I stumbled across an interesting article about a gene contributing to biofilm formation and drug tolerance. PA2146, a specific gene for Pseudomonas aeruginosa, produces a small protein that transcriptionally grows in amount during biofilm growth within PAO1, the most common lab strain used for research. This gene not only maintains structure during biofilm formation but also restores formation while protecting it from antimicrobial agents. Its involvement in gram-positive and negative species also determines its involvement with horizontal gene transfer– a process that transfers genetic material between individual cells of the same generation and strengthens biofilm against antibiotics. The methods they used to discover these results fascinated me; immunoblot analysis, immunoprecipitation procedures, electrophoresis protocols, and western blotting paired with antibodies were interesting processes with their complexities (Kaleta et al.).
What I find most interesting during my studies is the knowledge gap I have for these topics, given that I’m taking AP Biology next year. In this first edition of my molecular biology articles, I hope to educate myself through writing. Stay tuned for more science articles!
Work Cited
Parker, Nina, et al. 9.1 How Microbes Grow – Microbiology | OpenStax. 1 Nov. 2016, openstax.org/books/microbiology/pages/9-1-how-microbes-grow. Accessed 2025 April 7.
Sharma S, Mohler J, Mahajan SD, Schwartz SA, Bruggemann L, Aalinkeel R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms. 2023 Jun 19;11(6):1614.
doi: 10.3390/microorganisms11061614. Erratum in: Microorganisms. 2024 Sep 27;12(10):1961.
doi: 10.3390/microorganisms12101961. PMID: 37375116; PMCID: PMC10305407. Accessed 2025 April 7.
Kaleta, M.F., Petrova, O.E., Zampaloni, C. et al. A previously uncharacterized gene, PA2146, contributes to biofilm formation and drug tolerance across the ɣ-Proteobacteria. npj Biofilms Microbiomes 8, 54 (2022). https://doi.org/10.1038/s41522-022-00314-y